Dr. Crystal Rogers | Rogers Lab
Bio:
Dr. Crystal Rogers obtained a B.S. from UCLA in Organismal Biology, Ecology, and Evolution. She then received her PhD in Developmental Biology from Georgetown University under the mentorship of Dr. Elena Silva Casey and next, moved to a postdoctoral position at the California Institute of Technology in the Division of Biology and Biological Engineering in the lab of Dr. Marianne Bronner. In 2016, she became an Assistant Professor at California State University Northridge (CSUN) in the Biology Department where she focused on creating an undergraduate research program in developmental biology. In 2019, Dr. Rogers moved to the UC Davis School of Veterinary Medicine and is now an Associate Professor in the Department of Anatomy, Physiology, and Cell Biology. She was a 2023 UC Davis Chancellor's Fellowship For Diversity, Equity and Inclusion, and is currently the Chair of the Inclusion and Outreach Committee for the Society for Developmental Biology, and the Editor in Chief of the journal, Differentiation. Her lab studies the conserved and divergent molecular mechanisms that drive embryonic development in chicken, quail, peafowl and axolotl embryos.
Abstract:
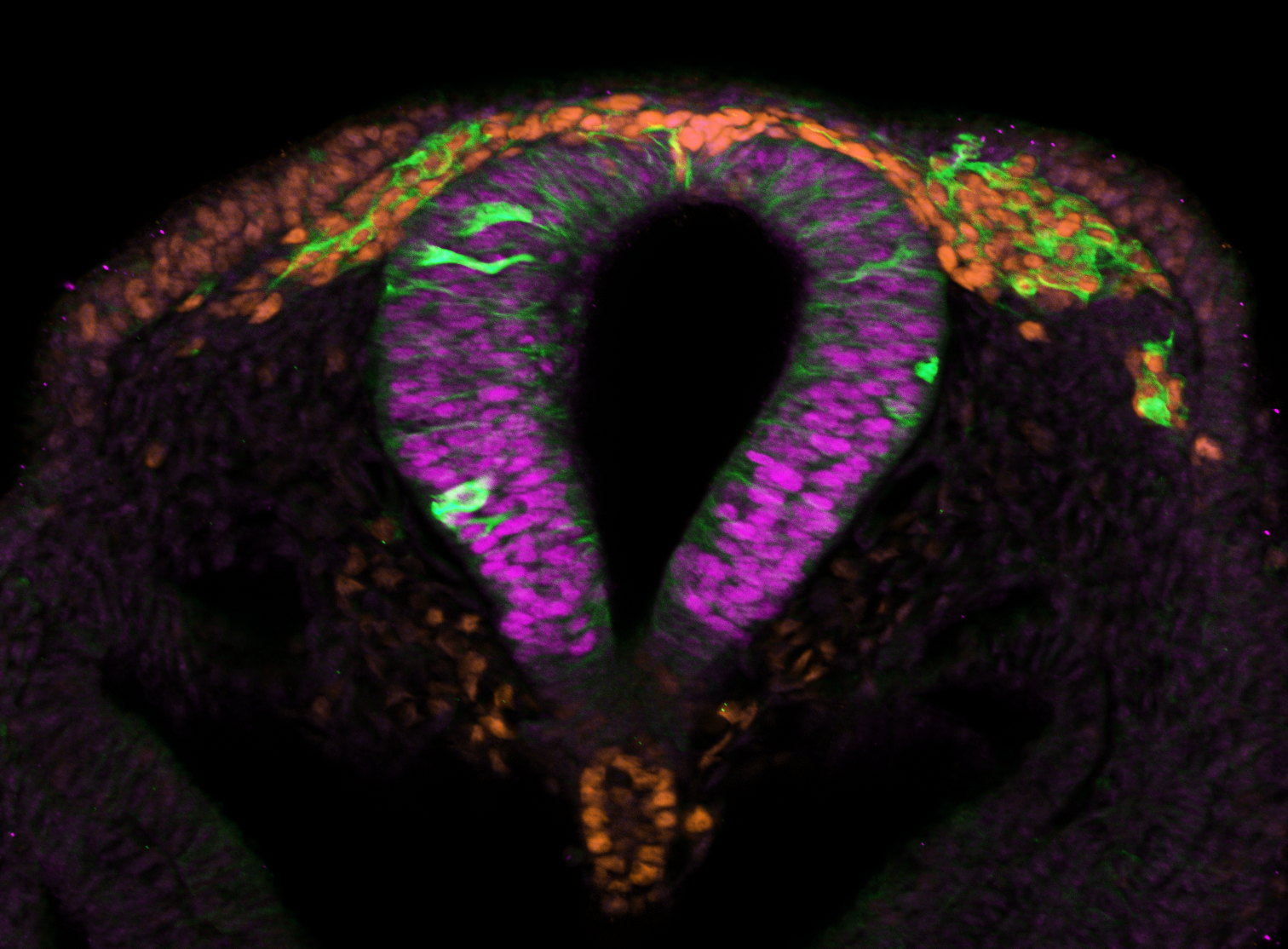
Neural crest cells are vertebrate-specific, pluripotent, embryonic stem cells that give rise to more
than 30 different adult cell and tissue types including pigment cells, craniofacial bone and cartilage, and the peripheral nervous system. Despite our extensive knowledge of the mechanisms governing neural crest development, which is primarily derived from a limited set of model organisms, there remains a crucial gap in confirming the functional conservation of proteins across species, encompassing their targets, interactions, and developmental roles. This challenge impedes a comprehensive understanding of neural crest evolution and development. Our research aspires to bridge this gap by identifying both conserved and divergent developmental mechanisms. We achieve this by characterizing the spatiotemporal expression patterns of key regulatory factors governing neural crest cell development and by perturbing these factors in quail (Coturnix japonica), chick (Gallus gallus), and peafowl (Pavo cristatus) embryos. Our findings reveal intriguing distinctions in the roles played by specific transcription factors at various developmental stages within closely related organisms. Moving forward, our focus will shift towards unraveling the intricate embryonic microenvironments responsible for shaping these distinct phenotypic outcomes. By investigating the factors influencing neural crest cell development across species, we aim to enrich our understanding of the evolutionary and developmental dynamics underlying this critical biological process.
Watch the seminar here!